For patients & families
Living with difficult-to-treat rheumatoid arthritis?
Rheumatoid arthritis (RA) can be painful, exhausting, and life-changing. When severalmedicines stop working or never work well, this is sometimes calledmulti-drug-resistant RA. MDR-RA is a European research project that aims to improvetreatment options and reduce trial-and-error for people in this situation.
Rheumatoid arthritis in everyday words
Rheumatoid arthritis is a long-term disease that causes pain, stiffness, and swelling in the joints. For many people, modern medicines can control the disease well. For others, several medicines do not work well enough, or stop working over time.
What does ‘multi-drug-resistant’ mean?
Multi-drug-resistant RA’ means that a person has already tried several standard treatments, often including biologic or targeted medicines, but their disease still stays active. This can lead to ongoing pain, fatigue, disability, and difficulty working or carrying out daily activities.
How many people are affected?
RA affects millions of people worldwide, including around 7 million in the EU. A significant group lives with multi-drug-resistant RA, facing higher health costs and a lower quality of life. MDR-RA focuses specifically on this group.
Why research is needed
Many people with multi-drug-resistant RA go through years of trial-and-error with medicines. MDR-RA aims to understand why treatments fail, identify early warning signs of resistance, and support more personalised treatment decisions so that the right medicine can be chosen sooner.
Our goal: fewer treatment failures
MDR-RA brings together hospitals, universities, patient organisations, and data experts across Europe. The shared goal is to reduce treatment failures in RA, protect mobility and independence, and cut the time and emotional cost of trying one medicine after another.
Two phases in everyday language
Phase 1 – Learning from real-life data
MDR-RA brings together hospitals, universities, patient organisations, and data experts across Europe. The shared goal is to reduce treatment failures in RA, protect mobility and independence, and cut the time and emotional cost of trying one medicine after another.
Phase 2 – Testing a new decision tool
Next, MDR-RA tests an AI-supported tool called iCare-RA in routine care. Doctors use this tool to help plan treatment for people with multi-drug-resistant RA, and their results are compared with usual care in a clinical trial. This shows whether the tool truly improves outcomes.
What this could mean for you
- Fewer ‘wait and see’ periods on medicines that are unlikely to work.
- More personalised treatment plans, based on your clinical history, imaging, and lab results.
- Better use of healthcare resources, so that effective care is more sustainable for health systems.
Who are Patient Research Partners?
Patient Research Partners (PRPs) are people living with RA who work as equal partners in the project. They help design study materials, comment on research plans, and check that information is clear and relevant. MDR-RA works with a diverse group of nine PRPs from several European countries.
The Patient Advisory Panel
Together, the PRPs form a Patient Advisory Panel. Each PRP is linked to one or more work packages, such as ethics, communication, health economics, or clinical research. This means patient voices are heard in project management, study design, and how results are shared.
How PRPs are already shaping MDR-RA
- Advising on how to make study information sheets easier to understand.
- Helping plan accessible meetings and events (breaks, rooms, formats).
- Giving feedback on iCare-RA so it reflects everyday life with RA in different countries.
Different ways people may take part
Some studies simply observe what happens in usual care and collect data over time. Others test new tools or care strategies, like the iCare-RA trial, which compares usual care with care supported by the decision tool. Not all studies are available in every country or centre. Reach out to us for more information.
Contact us through hunimed.eu
What might participation involve?
- Regular clinic visits to check your joints, symptoms, and medicines.
- Blood tests or imaging, such as ultrasound or MRI, to better understand your disease.
- Questionnaires about pain, fatigue, daily activities, and quality of life.
Your rights and data protection
Joining any study is voluntary. You can ask questions, take time to decide, andstop at any time without affecting your regular care. Data are handled according to strict European rules to protect your privacy and security.
Patient organisations working with MDR-RA
MDR-RA collaborates with patient organisations in many European countries.
They share project updates and offer information, support, and advocacy in your
language.
Learning more about RA
For broader information about RA, treatment options, and self-management, your national organisation or rheumatology society can provide trusted resources. Always talk to your healthcare team before changing any medication or treatment plan.
Get Involved
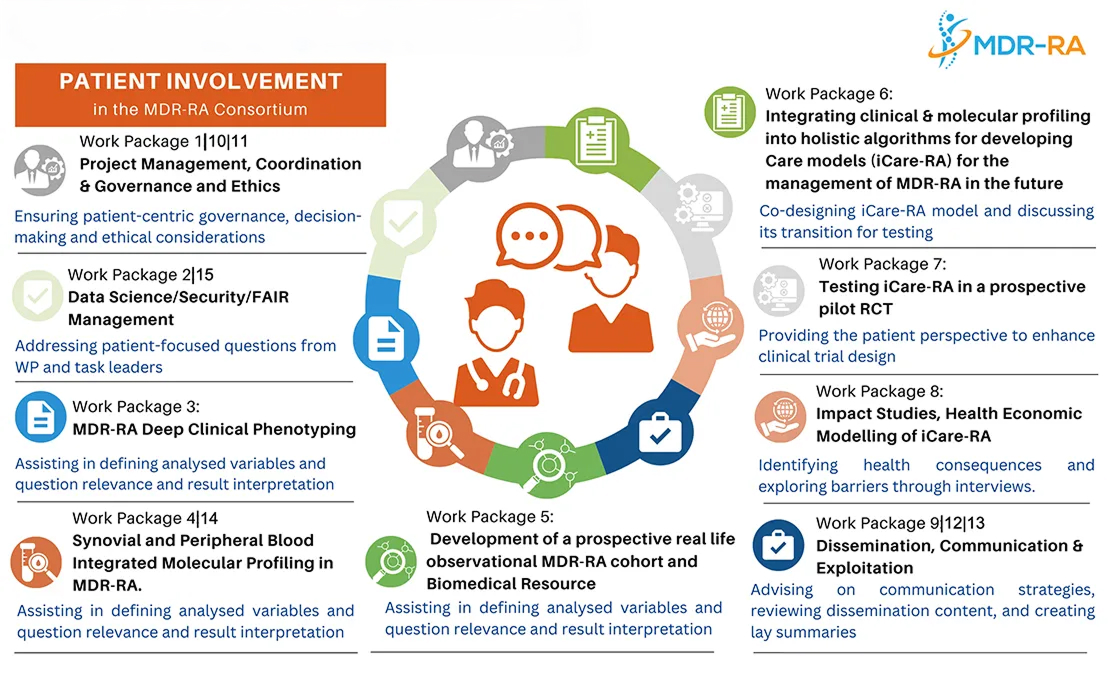
Patient Involvement and Engagement in the MDR-RA Project
Patient involvement and engagement is central to the success of MDR-RA, ensuring that the lived experiences and perspectives of people with rheumatoid arthritis guide every stage of our research and innovation.
From the beginning, the strategy was co-designed by EULAR, the Principal Investigator of the project, clinicians, and consortium partners to ensure that the patient voice is embedded in every stage of the research.
By making patients true partners in the project, MDR-RA aims to deliver solutions that are meaningful, practical, and impactful for those most affected.
Patient Involvement
From the very beginning, patients have played a central role in shaping our work. Nine Patient Research Partners (PRPs) joined the project through an open call launched by EULAR. They come from the EULAR-PARE PRP network, PARE member organisations, and consortium partners. Together, they represent different countries and bring lived experience of rheumatoid arthritis — ensuring that both personal and local perspectives are always part of the conversation. Some PRPs are also active in sister projects (SQUEEZE and STRATA-FIT), helping to strengthen a unified voice for the RA community.
To get started, we held dedicated meetings to clarify roles, responsibilities, and ways of working together. As part of onboarding, the Principal Investigator introduced the project in simple, accessible terms, giving PRPs a clear picture of the goals, partners, and expected impact.
How We Support Meaningful Involvement
To make sure PRPs can contribute effectively, we designed a tailored support process:
- Clear information: Work package leaders explained expectations, time commitments, and where patient input would be most valuable.
- Plain language: Summaries of each work package were provided in clear, accessible English.
- Matchmaking: EULAR helped align PRPs with the work packages most relevant to their interests and experience.
Following this, individual “meet-and-greet” sessions were held between PRPs and the respective work package leaders (or team members) to build connections and foster collaboration. To further support PRPs, EULAR created a dedicated package of resources containing valuable materials to facilitate their involvement. This package is continuously enriched with new content based on PRP feedback and emerging needs throughout the project.
Ongoing Roles for PRPs
- Our PRPs are actively involved in many aspects of the project, including:
- Clinical research and patient-facing documents
- Ethical committee contributions
- Communication and dissemination activities
- Co-creation of webinars
- Co-design of the iCare-RA model
- Health economics, including patient input on QALY assessments and alternative methods
Continuous Collaboration
To keep collaboration strong, EULAR organises regular meetings with PRPs, addressing challenges and ensuring good team dynamics. We also carry out regular evaluations of our patient involvement strategy — from both the PRP and consortium perspective — to keep improving and maximise the impact of this partnership
Patient Engagement
Alongside involvement in research, the project also developed a patient engagement strategy to strengthen communication and dissemination.
Fourteen national patient organisations from consortium countries were approached and invited to collaborate. In dedicated meetings with the principal investigator and EULAR, the project was explained in clear, accessible language, and organisations explored how they could support dissemination within their existing communication activities.
Who’s Involved: EULAR PARE and Other Patient Organisations
1 Österreichische Rheumaliga, Austria, Tanita-Christina Wilhelmer
2 ReumaNet vzw, Belgium, Nele Caeyers
3 Gigtforeningen, Denmark, Connie Ziegler
4 Deutsche Rheuma-Liga Bundesverband e.V., Germany, Dr. Jürgen Clausen
5 Associazione Nazionale Malati Reumatici ANMAR Onlus, Italy, Silvia Tonolo
6 National Association ReumaZorg Nederland, Netherlands, Gerardine Willemsen
7 Norsk Revmatikerforbund, Norway, Stine Dahl
8 Portuguese League Against Rheumatism, Portugal, Mariana Carvaho
9 Liga Reumatológica Española - LIRE, Spain,Marcos Séneca García Rodríguez
10 Reumatiker förbundet, Sweden, Stina Nordström
11 RLS- Rheumaliga Schweiz, Switzerland, Annette Stolz
12 Arthritis and Musculoskeletal Alliance ARMA, United Kingdom, Cathy Monaghan
13 The National Rheumatoid Arthritis Society, UK, Ailsa Bosworth & Peter Foxton
14 Versus Arthritis, United Kingdom, Pete Gowler
Together we agreed on:
- Quarterly project updates, written by EULAR and the Patient Advisory Panel, ensuring content remains patient-centred and easy to understand.
- Dissemination of updates through patient organisations at national level.
- Additional creative contributions, such as patient interviews about living with difficult-to-treat RA. These are translated into English and shared on the project website for the wider community.
Why It Matters
Difficult-to-treat rheumatoid arthritis is a severe condition that profoundly affects patients’ lives. By combining direct patient involvement in the research with active engagement of patient organisations, the MDR-RA project ensures its work remains relevant, visible, and meaningful to those it seeks to help.
This dual approach strengthens communication, builds awareness across countries, and ensures the project reflects the needs and hopes of the patient community.
“PRPs are not their diagnosis. They are individuals with unique experiences, and through their participation, they can widen the perspectives of research.” - Maryam Azimi (MDR-RA patient representative)